ICP-MS Instrumentation Guide for Newcomers
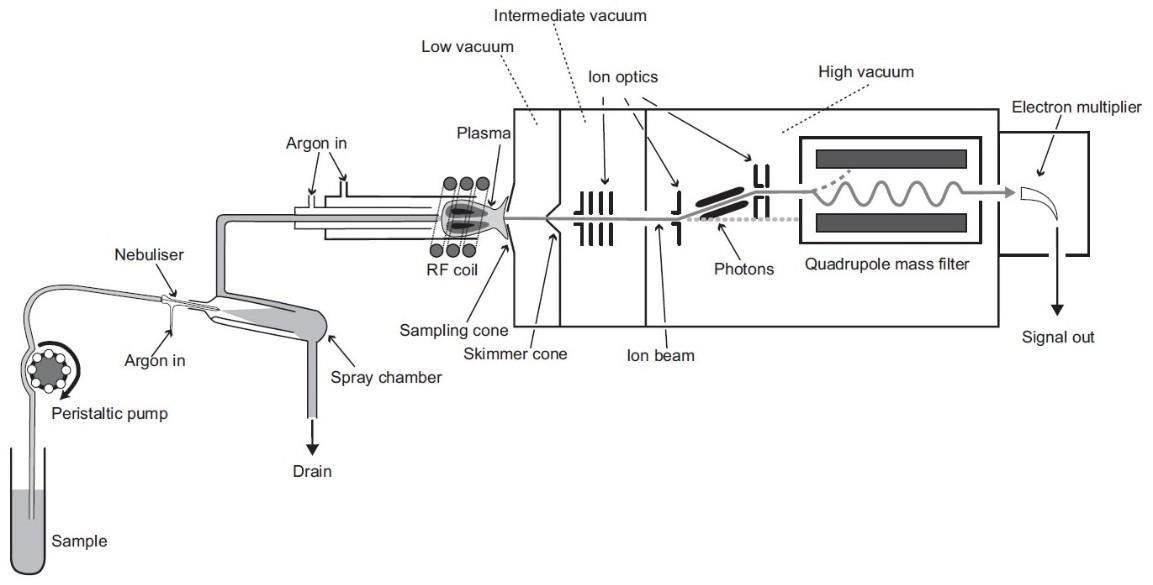
ICP-MS (Inductively Coupled Plasma Mass Spectrometry) is a sophisticated analytical technology that detects trace components in a variety of materials. It combines the ability of inductively coupled plasma (ICP) to atomize and ionize materials with mass spectrometry (MS) to evaluate the ions. ICP-MS is widely utilized in environmental testing, food safety, pharmaceutical analysis, and materials research due to its high sensitivity and precision. Understanding the components and operation of an ICP-MS system is critical for anybody hoping to use its capabilities for scientific research.
Key Components of an ICP-MS System
Sample Introduction System
The method for introducing samples into the ICP-MS is an essential component in the process of getting samples into the instrument. A nebulizer, a spray chamber, and a pump are often included in its components. The nebulizer is responsible for transforming the liquid sample into an aerosol, which is then delivered to the plasma. By removing larger droplets, the spray chamber helps to ensure that only fine mist is sent to the plasma torch, which is necessary for achieving the best possible ionization. Due to the fact that it guarantees sufficient sample delivery, this system has a direct impact on the sensitivity and accuracy of the analysis.
Ion Source & Plasma Torch
An inductively coupled plasma torch (ICP) serves as the ion source for an inductively coupled plasma mass spectrometer (ICP-MS). Atoms in the sample are ionized as a result of the plasma torch’s capacity to generate plasma at high temperatures. This plasma reaches temperatures of roughly 10,000 K, which provides enough energy for atomizing and ionizing elements. The ionization procedure is required to convert the material into charged particles that the mass analyzer can identify. The sensitivity of the ICP-MS system is completely determined by the efficacy of the ionization process.
Mass Analyzer & Detector
Using quadrupole or other kinds of mass spectrometers, the mass analyzer in ICP-MS separates ions according to their mass-to-charge ratio (m/z). The mass analyzer filters the ions before sending them on to the detector after ionization. The detector takes readings of the ion intensities associated with individual elements. It is possible to precisely quantify and identify trace elements in a sample using the ICP-MS technology, which does this by examining the ion intensities. Reliable findings must be obtained by using an accurate mass analyzer and detector.
ICP-MS Setup and Calibration Guide for Beginners
Basic Instrument Setup Checklist
The first step in setting up an ICP-MS apparatus is to ensure that all of the components are present and properly coupled. Conduct a comprehensive check to confirm that the mass analyzer, plasma torch, and sample introduction system are properly installed. Take the required actions to guarantee that the gas supply (oxygen, argon, etc.) is delivered at the appropriate flow rates. Examine the detector to make sure it’s calibrated and the vacuum system is working. By appropriately configuring the instrument, it is possible to establish consistent operation as well as accurate and reliable data collection.
Calibration Procedures for Accurate Results
Calibration is essential when using ICP-MS to obtain accurate results. You should start by using certified calibration standards that are the same as the sample matrix to ensure that the calibration curve appropriately reflects the actual conditions of the analysis. Make sure that the calibration of the instrument covers the proper concentration range for each element under observation. Performing a multi-point calibration is recommended in order to account for matrix effects and improve measurement accuracy. It is crucial to regularly assess the stability and performance following calibration in order to guarantee that the data stays true throughout the investigation.
Using Quality Control & Reference Standards
It is important to use reference standards and quality control (QC) to ensure that the results of an ICP-MS study are exact and correct. Samples that are considered quality control samples include duplicates, spiked samples, and blanks. The reason behind this is that these samples help detect any contamination or instrument drift. Comparisons between the results of a sample and the concentration of the reference standard are feasible because reference standards contain a known concentration of an element. It is significantly simpler to find any flaws or abnormalities in the ICP-MS analysis when quality control and reference standards are regularly implemented. This makes it easier to guarantee that the results are trustworthy.
Conclusion
Mastering ICP-MS requires understanding its components and the importance of correct setup and calibration. Users can get precise and reliable measurements for trace elements by handling sample introduction, ionization, and mass analysis effectively. If you want consistent, high-quality findings from your ICP-MS analyses, you must follow the correct calibration protocols and use quality control standards. Even novices may get impressive results using ICP-MS to analyze complicated materials as long as they pay close attention to detail and perform routine maintenance.

Samar
Punsuniverse — a realm crafted by me, Samar! You will find everything here that is related to puns, weather its food, animals, names or something elsse.